Alternative Games Called Chemistry
Available for BBC/Electron
A very comprehensive set of programs designed to test knowledge in preparation for the 1984/5 GCE/GCSE Chemistry exam. Programs should be used with the documentation included in the original pack.
| Genre: | General: Education |
| Publisher: | Letts |
| Cover Art Language: | English |
| Machine Compatibility: | BBC Model B, BBC Model B+, BBC Master 128, Acorn Electron |
| Release: | Professionally released on Cassette |
| Available For: | Amstrad CPC464, BBC Model B, BBC/Electron, Commodore 64, Commodore Vic 20, Dragon 32 & Spectrum 48K |
| Compatible Emulators: | BeebEm (PC (Windows)) PcBBC (PC (MS-DOS)) Model B Emulator (PC (Windows)) Elkulator 1.0 (PC (Windows)) |
| Original Release Date: | 2nd September 1985 |
| Original Release Price: | £11.50 |
| Market Valuation: | £2.50 (How Is This Calculated?) |
| Item Weight: | 64g |
| Box Type: | Cassette Single Plastic Clear |
| Author(s): | David R. Hopkins |
Variant Items
There are 0 other items featuring this same game (that we know about!). Click any of them for their details.
Active Auctions
Closed Auctions
Buy It
Unfortunately no-one is currently selling this item.
Auction Price Watch
Worried you're being ripped off? Closing prices on eBay can help you decide what a reasonable price is for a particular item.

Electron User
1st January 1986
It is fit for its purpose and pupils taking chemistry at 16+ levels could find it useful. Read Review

Home Computing Weekly
1st October 1985
This package would definitely be of benefit to the 'O' level student. Read Review
Full Instructions
Introduction
Computer-based techniques can lead a student to a clearer understanding of problem areas in a subject. The Letts Keyfacts Revision Software provides suites of programs specifically designed to develop insight into these areas of difficulty. Each package contains up to 10 interactive programs based on major topics found in all the current 16+ (O-Level GCE, CSE and GCSE) syllabuses.
As an integral part of a revision scheme the programs will assist understanding and reinforce learning. A variety of approaches is used to maximise the student's interest and to introduce an element of enjoyment of home study. Graphics are presented in an imaginative, interactive way as a positive teaching aid. An illustrated booklet provides information on how to get the most out of the programs.
Chemistry
Nine interactive programs, approved by subject teachers and tested by students are contained on the two cassette tapes. Topics included are:
- Atomic structure and bonds
- Periodic tabel
- Identificatin of substances
- Calculations
- Formulae and equations
- Electroysis
- Chemical apparatus
- Acids, bases and salts
- Reactions
- Organic compounds
The Programs (Program loading names in brackets)
The programs on these cassettes have been devised to make your revision more approachable and enjoyable. Organisation is the key to making the most of your revision time. For each subject follow these simple rules.
- Know your syllabus. A quick reference to the relevant syllabus analysis table to be found at the front of the Letts Study Aids Revise series is advised.
- Devise a timetable, as soon as the mocks are over, which will allow you to go through the syllabus at least twice (more for problem areas).
- For each topic, read all your available material - class notes and textbooks. Make summary notes as you go, then test yourself. Keyfacts Multiple Choice or Letts Study Aids Objective Questions will give invaluable practice and help. Finally, run the relevant computer program which will both test your knowledge and give you another perspective.
- Just before the examination, use all your summary notes to jog your memory and the whole subject program suite to reinforce your understanding.
Most programs have a 'help' facility. Do not 'over-use' it - use only when you cannot arrive at correct responses by your own efforts. In some cases the computer automatically helps you after 2 or 3 wrong attempts. In other cases you must request help by pressing a key (usually 'X' or '?' as indicated on screen).
When entering chemical names use terminology of the form 'iron (III) chloride' and not 'ferric chloride'. The use of the function keys for formulae is explained in this booklet for relevant programs.
The Programs
|
Tape 1
Side A Atomic structure & bonding ("ATOM") Periodic table ("PERTAB") Formulae & equations ("FORMRUL") |
Side B Chemical deducations ("DEDUCE") Electrolysis ("ELECT") |
|
|
Tape 2
Side A Apparatus 1 ("APPRUL") Apparatus 2 ("AP2RUL") |
Side B Acids bases & salts ("ABS") Organic chemistry 1 ("ORGCHEM") Organic chemistry 2 ("ORG) |
The Programs
1. Atomic Structure and Bonding ("ATOM")
This program is designed to develop your understanding of the atomic structure of elements and the relationships between atomic structure and different types of chemical bonds. This is achieved by the gradual completion of descriptive paragraphs about: atomic structure; ionic and metallic bonds; covalent and dative bonds; valency. You may select one of five levels of difficulty. The easiest requires you to enter only a few missing words whereas in the hardest you are given no words to help you. If you find any of the paragraphs too difficult you may use a built-in help facility. By entering 'X' you instruct the computer to complete the passage for you.
2. Periodic table ("PERTAB")
This program enables you to test your knowledge of the location in the periodic table of the more familiar elements. Alternatively you may select options which require you to apply your understanding of the electronic configuration of atoms. You may select how many elements you wish to predict and also choose between options of different levels of difficulty. The less difficult option is confined to those elements with which you should be familiar wereas the more difficult option extends your understanding to less familiar elements. You will need to know the sequence in which electrons are added to successive elements for both main group and transition elements.
3. Formulae and equations ("FORMRUL")
Chemistry is primarily concerned with the interaction of materials. Reactions are normally represented by balanced equations in which the reactants and products are represented by chemical formulae. You may select between completing equations in which both sides are incomplete or organising a selection of chemicals to produce a valid balanced equation. Both selections have built-in help facilities. The entry of correct formulae requires the correct use of upper and lower case letters. Additionally, you will need to use subscript numbers. These have been located on the programmable function keys of the same number. It is suggested that you set your keyboard for lower case letters and use the SHIFT key for capitals as with a typewriter. For example for Na2SO4, use keys as follows:
SHIFT-N a f2 SHIFT-S SHIFT-O f4
4. Chemical deductions ("DEDUCE")
This will test your overall knowledge of chemistry. The computer selects a chemical substance randomly from a databank. For each substance, you will be provided with five clues, one at a time, from which you are asked to identify the selected substance. Some clues are numerical. For these you will need a calculator and a table of atomic masses. At any stage, you may enter your answer. If it is incorrect, the next clue will automatically appear. When each substance has been identified, or revealed by the computer, you will be asked for its chemical formula. Use the keyboard and function keys as demonstrated in the previous program. A help facility is provided.
5. Electrolysis ("ELECT")
A random combination of electrolytes, molten or in dilure aqueous solution, is selected by the computer. You are required to identify the ions present, to which electrode they migrate and to identify the preferred ion reaction at each electrode. Finally you are requested to compile the ionic equation for each electrode reaction. Only inert electrode systems are considered.
You will need to use subscript and superscript numerals for chemical formulae and ions. The function keys are programmed for this and on-screen information tells you which keys to use. A help facility is provided for the construction of ionic equations.
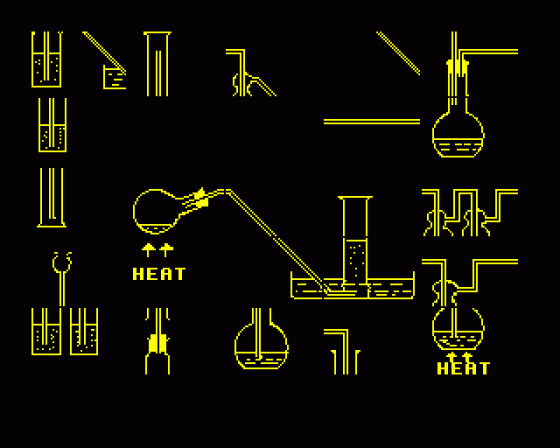
6. Apparatus 1 ("APPRUL")
This is a jigsaw program. You have a choice from thirteen different gases and must construct the apparatus which is used for the laboratory preparation. The screen is divided into 25 boxes, each box containing a piece of the jigsaw. The correct apparatus must be constructed in the central nine boxes, using the full width of these. The jigsaw pieces are moved by 'swapping' boxes.
This is done by identifying the letters for the boxes to be swapped. For example P M will swap the contents of box P and box M. You may discard the contents of a box with the '/' key. For example / B will empty box B. Once a piece of jigsaw has been discarded, it cannot be recalled. When the picture is correct, extraneous pieces of picture are removed by the computer and you are requested to enter both the labels and the equation. Help is available on request in all stages of the program with the '?' key. Use the function keys for subscripts as demonstrated previously.
7. Apparatus 2 ("AP2RUL")
A random selection of laboratory apparatus for the preparation of gases produces one of the completed pictures from the previous program but containing at least one error in the picture. As each incorrect part of apparatus is identified it will be removed from the screen, leaving a blank box. When you have identified all errors, the picture will be redrawn correctly. The correct picture will contain at least one labelling error, each of which must be identified one at a time.
8. Acids Bases & Salts ("ABS")
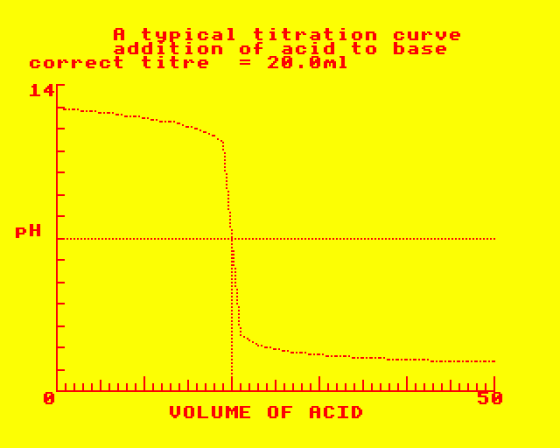
This program is in three parts. In the first, you are required to complete descriptive passages about acids, bases, salts and pH. In the second part you are asked to comment on the proposed reactions, represented by equations. All are related to the various methods of preparing salts and their properties. The third part demonstrates how pH changes in acid-base titrations and identifies the pH end-point. You may enter your own data and compare your result with that calculated by the computer.
9. Organic Chemistry 1 ("ORGCHEM")
This consists of four descriptive passages about the structure of organic molecules. You are required to complete them, with different levels of difficulty as in some previous programs. When each passage is completed, diagrams are produced which illustrate the substance of the passage.
10. Organic Chemistry 2 ("ORG")
The program requires you to identify isomers, homologues and compounds with similar reactivity from a selection of twelve formulae displayed on screen. This tests your powers of observation rather than any detailed knowledge of the properties of organic molecules. If your press '?' the computer will solve the problem for you.
Screen Designers
The following utilities are also available to allow you to edit the supplied screens of this game:
Cheats
Download
A digital version of this item can be downloaded right here at Everygamegoing (All our downloads are in .zip format).
| Download | What It Contains |
|---|---|
| A digital version of Chemistry suitable for BeebEm (PC (Windows)), PcBBC (PC (MS-DOS)), Model B Emulator (PC (Windows)), Elkulator 1.0 (PC (Windows)) |
Report A Problem
We thank you from the bottom of our hearts if you report something wrong on our site. It's the only way we can fix any problems!
You are not currently logged in so your report will be anonymous.
Add Note
Release Country
Change the country to update it. Click outside of this pop-up to cancel.
Scan Of Selected Article
If you auction an item, it will no longer show in the regular shop section of the site.













